When Gina noticed her daughter Francesca wasn’t hitting her gross motor milestones, she turned to...
Read MoreEffective Monday, January 5th, Inspira Health is now at Yellow Alert Status: Masks for staff, patients and visitors in all high-risk areas across our facilities are strongly recommended.

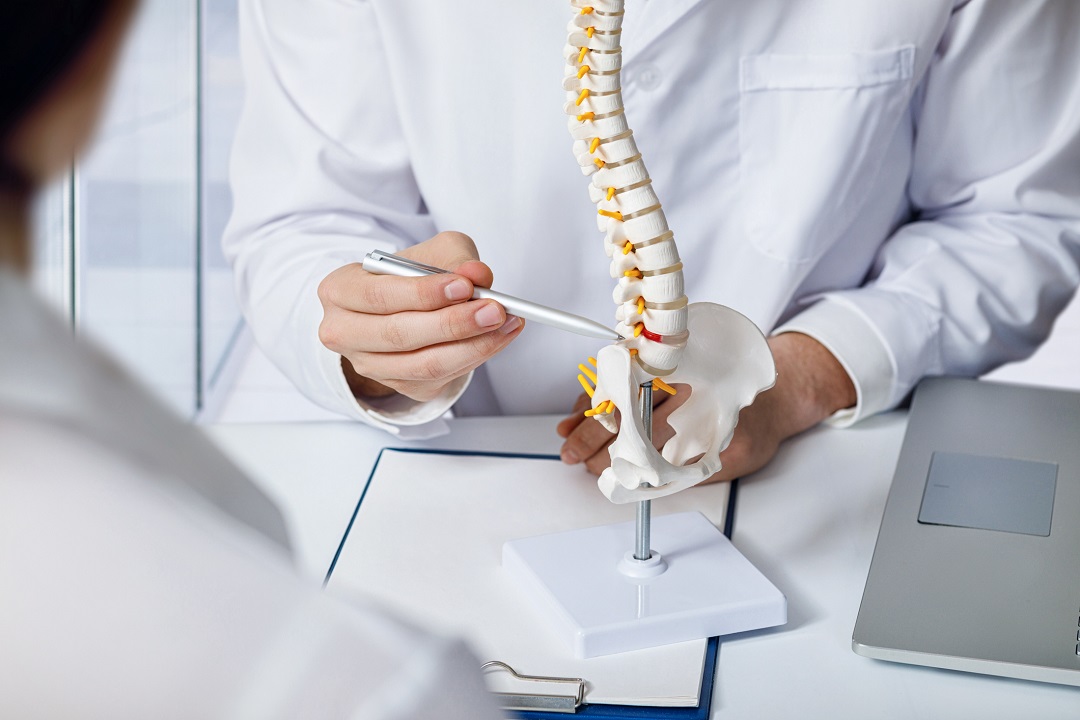
Herniated discs are the leading cause of neck, back and leg pain for people in the United States. While a lumbar disc herniation can be treated successfully with a diskectomy procedure, those who have a large defect have a 30 percent chance of reherniation after treatment. Inspira Health is among the first health systems in New Jersey to offer a new treatment option for those at high risk of reherniation.
The Barricaid annular closure device is an implant that closes large holes in the disc walls of patients undergoing diskectomies. Approved by the FDA in March 2020, the device has been implanted in approximately 8,500 patients worldwide and has been proven 95 percent effective in preventing reoperations due to reherniation.
If you have a patient with a lumbar disc herniation or sciatica that isn’t responding to treatments such as anti-inflammatory medication and physical therapy, they may be a candidate for a diskectomy. “Disc herniation is treated according to the severity of the symptoms, meaning not all patients will be candidates for surgery,” said R.J. Meagher, M.D., spine and neurosurgery specialist at Princeton Brain, Spine and Sports Medicine and staff neurosurgeon at Inspira Health. “But if a patient’s pain is affecting their quality of life, and it’s not getting better with less invasive treatments, you should refer them to a spine surgeon to explore their options.”
In addition, not all patients are candidates for the Barricaid device. Indications for device insertion depend on each person’s particular anatomy, and the decision to use the device is made intraoperatively by the surgeon during a minimally invasive diskectomy procedure.
The procedure to implant the Barricaid device usually takes less than an hour. During the procedure, a small titanium anchor is implanted into the bone while a polyester flap forms a barrier to block the hole in the disc. As with any procedure, there are some risks, including the risk of nerve amputation or the device coming loose.
“It’s important to know that this permanent device doesn’t eliminate reherniation, but reduces the risk of it occurring,” said Dr. Meagher.
To learn more about Inspira’s innovative treatment options for spine conditions or to help your patient better understand their candidacy for the Barricaid procedure, visit www.InspiraHealthNetwork.org/Barricaid. Or to consult with Dr. Meagher about a patient’s case, call his office at (610) 915-2110.
As a high reliability organization (HRO), Inspira is committed to the safety of all patients, staff and providers.

When Gina noticed her daughter Francesca wasn’t hitting her gross motor milestones, she turned to...
Read More
Sports medicine and physical therapy both play vital roles in injury recovery, but they serve...
Read More
Whether you’re a weekend warrior or a seasoned competitor, a few strength and mobility tips can go a...
Read More